Your cart is currently empty!
Opioid receptors
Opioid receptors (opiate receptors) are a type of receptors of the nervous system related to G protein coupled receptors. Their main function in the body is to regulate pain. Currently, there are four main groups of opioid receptors: μ- (mu), δ-(delta), κ-(kappa) and nociceptive receptors. They bind to both endogenous (produced in the body) and exogenous (externally supplied) opioid ligands. Opiate receptors are widespread in the brain, spinal cord, as well as in the gastrointestinal tract and other organs.
History
By the mid-1960s, analyzing the results of pharmacological studies, scientists began to assume that opioids probably act on specific receptors. The receptors were first identified as specific molecules during studies in which it was found that opiates labeled with radioisotopes bind to subcellular fractions of the brain. The first such study was published in 1971 using 3H-levorphanol and its antagonist naloxone. In 1973, Candice Perth (eng.) and Solomon Snyder. The results of the first detailed radioisotope study of opioid receptors using 3H-naloxone have been published. This study was recognized as the first accurate detection of opioid receptors, although two other similar studies were conducted shortly after it in the same year. In 1976, Martin and colleagues concluded from in vivo studies in dogs that there are several types of opioid receptors. To confirm their existence, attempts were made to isolate the purified protein of opioid receptors, but they were unsuccessful. In the early 1990s, molecular biological research elucidated the structure and mechanism of action of opioid receptors. Four different cDNAs have been identified as members of the opioid receptor family. Three of them correspond to µ-, δ- and κ-receptors, and the fourth corresponds to a new type of receptor — nociceptive or ORL—1 (English opioid-receptor-like 1), which is also classified as an opioid, although it does not have a high affinity for opioid ligands.
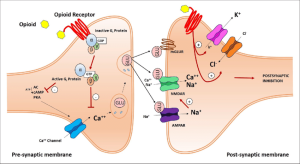
Mechanism of action
The mechanism of inhibition of the transmission of pain impulses in the NMDA synapse by means of m-opioid receptors
When the opioid receptor is activated, adenylate cyclase is inhibited, which plays an important role in the synthesis of the secondary cAMP mediator (cAMP), and ion channels are regulated. Closure of potential-dependent calcium channels in the presynaptic neuron leads to a decrease in the release of excitatory neurotransmitters (such as glutamic acid), and activation of potassium channels in the postsynaptic neuron leads to hyperpolarization of the membrane, which reduces the sensitivity of the neuron to excitatory neurotransmitters.
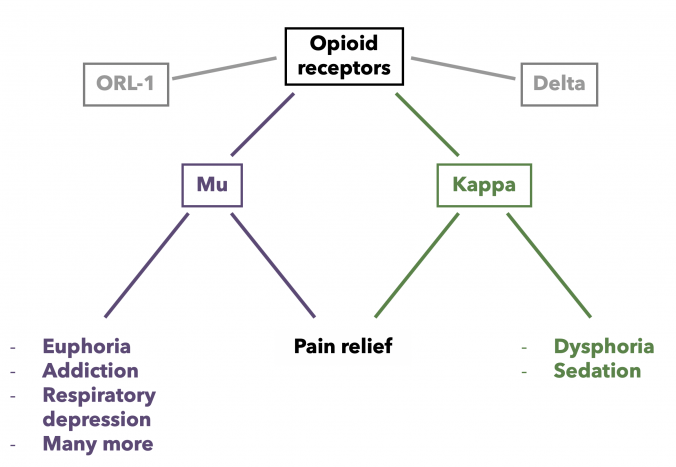
Types of opioid receptors
Currently, there are four main groups of opioid receptors, each of which is further subdivided into several subtypes:
MOP:
μ1,
μ2,
μ3,
delta (δ)
DOP δ1, δ2,
kappa (k)
KOP k1, k2, k3,
The
nociceptin NOP receptor.
The effect of analgesia is observed with stimulation of the μ-, δ- and κ-receptors. In addition, m-receptor agonists cause respiratory depression and sedation, while k-receptor agonists cause psychotomimetic effects. The action of most opioid analgesics is associated with the stimulation of m-type receptors.
Nomenclature
Opioid receptors were named after the first letter of the ligand with which their connection was first discovered. Thus, morphine was the first substance to have the ability to bind to m-receptors, and the k-receptors are named after the discovery of their binding to ketocyclazocin. A receptor with high affinity for enkephalin was also found in the vas deferens of mice and named the δ-receptor. Later, another opioid receptor was discovered and cloned based on cDNA homology. This receptor is known as the nociceptin receptor or ORL 1 receptor.
The existence of separate subtypes of opioid receptors in human tissues has been suggested, but researchers have not yet been able to obtain genetic or molecular evidence of their existence and believe that they arise as a result of posttranslational modification of cloned receptor types or dimerization.
The IUPHAR Subcommittee It allows the use of the generally accepted Greek classification, but recommends that the 3 classical receptors (μ-, δ-, κ-) and the nociceptin receptor be designated as MOP, DOP, KOP and NOP, respectively.
Endogenous opioid peptides are produced in the body itself and realize their opioid effects. The discovery of opioid receptors led to the discovery of their endogenous ligands. Initially, three families of opioid receptors (endorphins, enkephalins, and dynorphins) were found in various regions of the central nervous system, the gastrointestinal tract, and other peripheral tissues. Later, nociceptins, endomorphins, and other opioid peptides were discovered. At the same time, endorphins and endomorphins exhibit maximum affinity for the type m receptors, enkephalins — type δ, and dynorphins — type k.
Exogenous opioids enter the body from the outside and bind to opioid receptors. The first opioid discovered was morphine, an alkaloid of the opium poppy, isolated by Friedrich Certurner from opium in 1804. Currently, a large number of compounds (both morphine derivatives and substances of other structures) that are ligands to opioid receptors are known. Natural, synthetic, and semi-synthetic opioids are distinguished by origin. Many of them are used in medicine as analgesics and cough suppressants.
Mk-opioid receptor agonists have a high potential for abuse, causing euphoria in the short term, and with systematic use, severe physical and mental dependence. For this reason, opioid trafficking is controlled in most countries.
Heroin itself has a rather weak affinity for m-receptors, but it easily penetrates the blood-brain barrier, where it is converted into 6-monoacetylmorphine, a powerful m—receptor agonist.
Other receptors
σ-receptors were previously classified as opioids, since it was believed that the antitussive effect of many opioids is realized through action on these receptors, and the first selective σ-agonists were opioid derivatives (for example, allylnormethazocine). However, it was found that σ-receptors are not activated by endogenous opioid peptides and differ greatly from other opioid receptors in both function and genetic structure. They also showed high sensitivity to phencyclidine and ketamine, which are specific antagonists of the glutamate-N-methyl-D-aspartate complex. In addition, they do not undergo naloxone reversal and are stereoselective to right-handed isomers, while opioid receptors are selective to left-handed isomers.
Other opioid receptors have also been suggested due to the discovery of effects of endogenous opioid peptides that are not mediated by one of the four known opioid receptors. Only one of these receptors was discovered and named the zeta (ζ-) opioid receptor, which manifested itself as a modulator of cell growth factor under the action of its endogenous ligand, met-enkephalin. This receptor is now more commonly referred to as the opioid growth factor receptor (OGFr).
The epsilon (ε-) opioid receptor is assumed to exist. This assumption appeared after beta-endorphin had effects that were not mediated by any known opioid receptor. Activation of the ε-receptor causes marked analgesia and release of met-enkephalin, and a number of widely used opioid agonists, such as the μ-agonist etorphine and the κ-agonist bremazocine, have been found to act as agonists of these effects (even in the presence of antagonists to their more well-known targets), and buprenorphine acts as an antagonist of this receptor. Currently, several selective agonists and antagonists of the putative e-receptors are available, but attempts to detect the gene for these receptors have been unsuccessful.
Pathology
A118G mutation (replacement of adenine by guanine in exon 1), which leads to the substitution of asparagine at position 40 for aspartate (N40D), is the most common mutation leading to a change in the gene product of the human µ-opioid receptor. It is assumed that patients with oncological diseases homozygous for the A118G variant require a higher dose of morphine during long-term treatment of pain syndrome. Also, patient-controlled intravenous morphine administration after total hysterectomy was significantly higher in women homozygous for variant A118G than in other patients. Some forms of δ-opioid receptor mutations lead to permanent receptor activation.

Leave a Reply